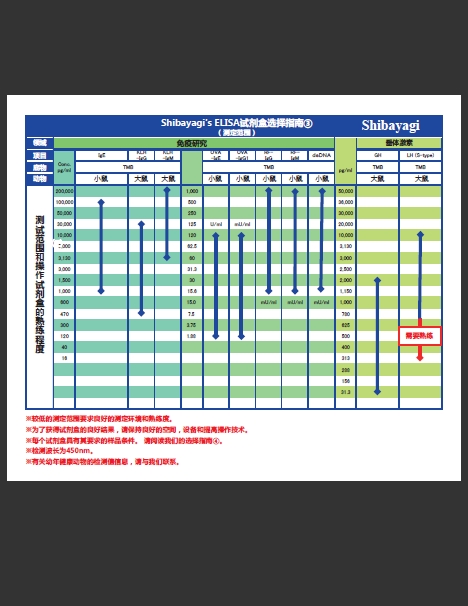
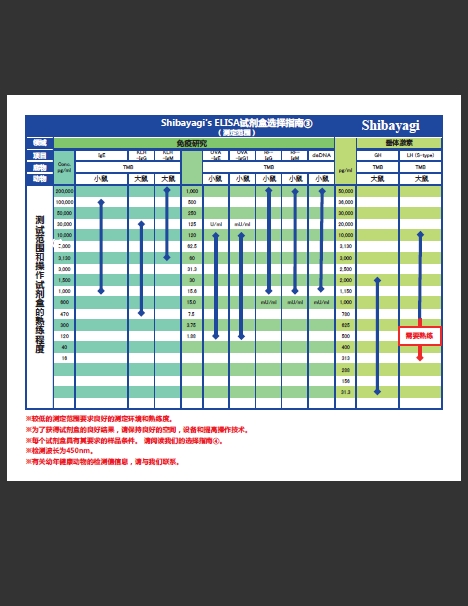
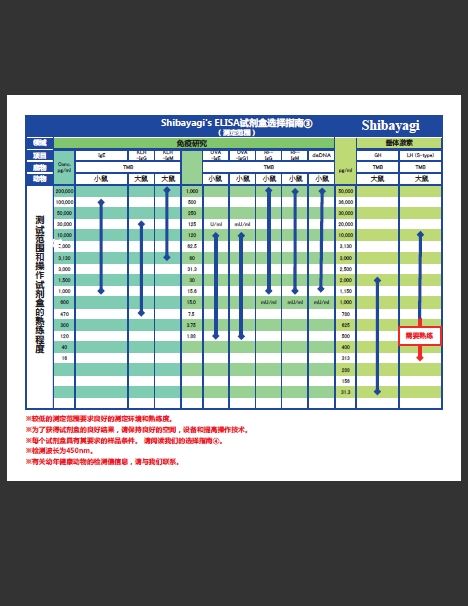
|
1.
|
Endoplasmic Reticulum Stress in Mice Increases Hepatic Expression of Genes Carrying a Premature Termination Codon via a Nutritional Status-Independent GRP78-Dependent Mechanism.Harada N, Okuyama M, Yoshikatsu A, Yamamoto H, Ishiwata S, Hamada C, Hirose T, Shono M, Kuroda M, Tsutsumi R, Takeo J, Taketani Y, Nakaya Y, Sakaue H.J Cell Biochem. 2017 Nov;118(11):3810-3824.
|
|
2.
|
Effects of β-Glucan Content and Pearling of Barley in Diet-Induced Obese MiceSeiichiro Aoe, Yasunori Ichinose, Noriko Kohyama, Kozo Komae, Asuka Takahashi, Toji Yoshioka, and Takashi Yanagisawa.Posted online on 27 Sep 2017. https://doi.org/10.1094/CCHEM-04-17-0083-R
|
|
3.
|
10-Hydroxy-2-decenoic acid, a natural product, improves hyperglycemia and insulin resistance in obese/diabetic KK-Ay mice, but does not prevent obesity Risa WATADANI, Jun KOTOH, Daiki SASAKI, Azusa SOMEYA, Kozo MATSUMOTO, Akihiko MAEDA Journal of Veterinary Medical Science, Vol. 79 (2017) No. 9 Sept, p.1596-1602
|
|
4.
|
S-Equol Activates cAMP Signaling at the Plasma Membrane of INS-1 Pancreatic β-Cells and Protects against Streptozotocin-Induced Hyperglycemia by Increasing β-Cell Function in Male Mice. Horiuchi H, Usami A, Shirai R, Harada N, Ikushiro S, Sakaki T, Nakano Y, Inui H, Yamaji R. J Nutr. 2017 Sep;147(9):1631-1639.
|
|
5.
|
Pathological and gene expression analysis of a polygenic diabetes model, NONcNZO10/LtJ mice. Hirata T, Yoshitomi T, Inoue M, Iigo Y, Matsumoto K, Kubota K, Shinagawa A. Gene. 2017 Sep 20;629:52-58.
|
|
6.
|
Suppression of GRK2 expression reduces endothelial dysfunction by restoring glucose homeostasis. Taguchi K, Hida M, Hasegawa M, Narimatsu H, Matsumoto T, Kobayashi T. Sci Rep. 2017 Aug 16;7(1):8436.
|
|
7.
|
Gender difference in NASH susceptibility: Roles of hepatocyte Ikkβ and Sult1e1. Matsushita N, Hassanein MT, Martinez-Clemente M, Lazaro R, French SW, Xie W, Lai K, Karin M, Tsukamoto H. PLoS One. 2017 Aug 10;12(8):e0181052.
|
|
8.
|
Intake of mulberry 1-deoxynojirimycin prevents colorectal cancer in mice. E S, Yamamoto K, Sakamoto Y, Mizowaki Y, Iwagaki Y, Kimura T, Nakagawa K, Miyazawa T, Tsuduki T. J Clin Biochem Nutr. 2017 Jul;61(1):47-52.
|
|
9.
|
Sake lees extract improves hepatic lipid accumulation in high fat diet-fed mice. Kubo H, Hoshi M, Matsumoto T, Irie M, Oura S, Tsutsumi H, Hata Y, Yamamoto Y, Saito K Lipids Health Dis. 2017 Jun 3;16(1):106.
|
|
10.
|
Moringa oleifera from Cambodia Ameliorates Oxidative Stress, Hyperglycemia, and Kidney Dysfunction in Type 2 Diabetic Mice. Tang Y, Choi EJ, Han WC, Oh M, Kim J, Hwang JY, Park PJ, Moon SH, Kim YS, Kim EK. J Med Food. 2017 May;20(5):502-510.
|
|
11.
|
4μ8C Inhibits Insulin Secretion Independent of IRE1α RNase Activity. Sato H, Shiba Y, Tsuchiya Y, Saito M, Kohno K. Cell Struct Funct. 2017 May 3;42(1):61-70
|
|
12.
|
Inhibition of Gastric Inhibitory Polypeptide Receptor Signaling in Adipose Tissue Reduces Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice. Joo E, Harada N, Yamane S, Fukushima T, Taura D, Iwasaki K, Sankoda A, Shibue K, Harada T, Suzuki K, Hamasaki A, Inagaki N. Diabetes. 2017 Apr;66(4):868-879.
|
|
13.
|
Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models. Kim MK, Chae YN, Ahn GJ, Shin CY, Choi SH, Yang EK, Sohn YS, Son MH Arch Pharm Res. 2017 Feb;40(2):268-281.
|
|
14.
|
Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. Mei YOSHIDA, Kaori HAYASHI, Risa WATADANI, Yoshiyasu OKANO, Keiya TANIMURA, Jun KOTOH, Daiki SASAKI, Kozo MATSUMOTO, Akihiko MAEDA Journal of Veterinary Medical Science, Vol. 79 (2017) No. 2 February p. 299-307
|
|
15.
|
PDK1-FoxO1 pathway in AgRP neurons of arcuate nucleus promotes bone formation via GHRH-GH-IGF1 axis. Sasanuma H, Nakata M, Parmila K, Nakae J, Yada T. Mol Metab. 2017 Feb 17;6(5):428-439.
|
|
16.
|
Soy Protein Isolate Suppresses Lipodystrophy-induced Hepatic Lipid Accumulation in Model Mice. Nagao K, Matsumoto A, Kai S, Kayashima T, Yanagita T. J Oleo Sci. 2017 Feb 1;66(2):161-169.
|
|
17.
|
A high-fat diet temporarily renders Sod1-deficient mice resistant to an oxidative insult.
Ito J, Ishii N, Akihara R, Lee J, Kurahashi T, Homma T, Kawasaki R, Fujii J J Nutr Biochem. 2017 Feb;40:44-52.
|
|
18.
|
Macrophage ubiquitin-specific protease 2 modifies insulin sensitivity in obese mice. Saito N, Kimura S, Miyamoto T, Fukushima S, Amagasa M, Shimamoto Y, Nishioka C, Okamoto S, Toda C, Washio K, Asano A, Miyoshi I, Takahashi E, Kitamura H. Biochem Biophys Rep. 2017 Jan 26;9:322-329.
|
|
19.
|
Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. Watanabe H, Inaba Y, Kimura K, Asahara SI, Kido Y, Matsumoto M, Motoyama T, Tachibana N, Kaneko S, Kohno M, Inoue H. J Nutr. 2017 Jan;147(1):52-60.
|
|
20.
|
BCL11B gene heterozygosity causes weight loss accompanied by increased energy consumption, but not defective adipogenesis, in mice Jun Inoue, Yusuke Ihara, Daisuke Tsukamoto, Keisuke Yasumoto, Tsutomu Hashidume, Kenya Kamimura, Shigeki Hirano, Makoto Shimizu, Ryo Kominami & Ryuichiro Sato Bioscience, Biotechnology, and Biochemistry, Vol.81, 2017,Issue 5, p922-930
|
|
21.
|
Macrophage ubiquitin-specific protease 2 modifies insulin sensitivity in obese mice. Natsuko Saitoa,Shunsuke Kimurac,Tomomi Miyamotod,Sanae Fukushimae,Misato Amagasaa,Yoshinori Shimamotob, Chieko Nishiokae,Shiki Okamotof,Chitoku Todag,Kohei Washioa,Atsushi Asanoh,Ichiro Miyoshid,Eiki Takahashie,Hiroshi Kitamura Biochemistry and Biophysics Reports,Volume 9, March 2017, Pages 322-329
|
|
22.
|
Agmatine ameliorates type 2 diabetes induced-Alzheimer’s disease-like alterations in high-fat diet-fed mice via reactivation of blunted insulin signalling. Kang S, Kim CH, Jung H, Kim E, Song HT, Lee JE. Neuropharmacology. 2017 Feb;113(Pt A):467-479
|
|
23.
|
Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high fat diet and ovariectomy in mice. Ohtake K, Ehara N, Chiba H, Nakano G, Sonoda K, Ito J, Uchida H, Kobayashi J. Am J Physiol Endocrinol Metab. 2017 Feb 14
|
|
24.
|
A high-fat diet temporarily renders Sod1-deficient mice resistant to an oxidative insult. Ito J, Ishii N, Akihara R, Lee J, Kurahashi T, Homma T, Kawasaki R, Fujii J. J Nutr Biochem. 2017 Feb;40:44-52.
|
|
25.
|
Inhibition of Gastric Inhibitory Polypeptide Receptor Signaling in Adipose Tissue Reduces Insulin Resistance and Hepatic Steatosis in High Fat Diet-Fed Mice. Joo E, Harada N, Yamane S, Fukushima T, Taura D, Iwasaki K, Sankoda A, Shibue K, Harada T, Suzuki K, Hamasaki A, Inagaki N. Diabetes. 2017 Jan 17.
|
|
26.
|
BCL11B gene heterozygosity causes weight loss accompanied by increased energy consumption, but not defective adipogenesis, in mice. Inoue J, Ihara Y, Tsukamoto D, Yasumoto K, Hashidume T, Kamimura K, Hirano S, Shimizu M, Kominami R, Sato R. Biosci Biotechnol Biochem. 2017 Jan 9:1-9.
|
|
27.
|
Attenuated secretion of glucose-dependent insulinotropic polypeptide (GIP) does not alleviate hyperphagic obesity and insulin resistance in ob/ob mice. Satoko Shimazu-Kuwahara,Norio Harada,Shunsuke Yamane,Erina Joo,Akiko Sankoda,Timothy J. Kieffer,Nobuya Inagaki. Molecular Metabolism,Available online 19 January 2017
|
|
28.
|
Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. Watanabe H, Inaba Y, Kimura K, Asahara SI, Kido Y, Matsumoto M, Motoyama T, Tachibana N, Kaneko S, Kohno M, Inoue H. J Nutr. 2017 Jan;147(1):52-60.
|
|
29.
|
Germinated Pigmented Rice (Oryza Sativa L. cv. Superhongmi) Improves Glucose and Bone Metabolisms in Ovariectomized Rats. Chung SI, Ryu SN, Kang MY. Nutrients. 2016 Oct 21;8(10).
|
|
30.
|
Angiopoietin-like peptide 4 regulates insulin secretion and islet morphology. Kim HK, Kwon O, Park KH, Lee KJ, Youn BS, Kim SW, Kim MS. Biochem Biophys Res Commun. 2017 Feb 7
|
|
31.
|
Ubc13 haploinsufficiency protects against age-related insulin resistance and high-fat diet-induced obesity. Joo E, Fukushima T, Harada N, Reed JC, Matsuzawa SI, Inagaki N. Sci Rep. 2016 Oct 31;6:35983.
|
|
32.
|
Procyanidin Promotes Translocation of Glucose Transporter 4 in Muscle of Mice through Activation of Insulin and AMPK Signaling Pathways. Yamashita Y, Wang L, Nanba F, Ito C, Toda T, Ashida H. PLoS One. 2016 Sep 6;11(9):e0161704
|
|
33.
|
Oxytocin Protects against Stress-Induced Cell Death in Murine Pancreatic β-Cells. Watanabe S, Wei FY, Matsunaga T, Matsunaga N, Kaitsuka T, Tomizawa K. Sci Rep. 2016 May 4;6:25185.
|
|
34.
|
Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Honma K, Hikosaka M, Mochizuki K, Goda T. Metabolism. 2016 Apr;65(4):482-91
|
|
35.
|
Sodium alginate prevents progression of non-alcoholic steatohepatitis and liver carcinogenesis in obese and diabetic mice. Miyazaki T, Shirakami Y, Kubota M, Ideta T, Kochi T, Sakai H, Tanaka T, Moriwaki H, Shimizu M. Oncotarget. 2016 Mar 1;7(9):10448-58.
|
|
36.
|
Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M, Yagi S, Soeki T, Hayashi T, Imoto I, Sakaue H, Shimabukuro M, Sata M. Sci Adv. 2016 Mar 25;2(3):e1501332
|
|
37.
|
Fatty Liver and Insulin Resistance in the Liver-Specific Knockout Mice of Mitogen Inducible Gene-6. Park BK, Lee EA, Kim HY, Lee JC, Kim KS, Jeong WH, Kim KY, Ku BJ, Rhee SD. J Diabetes Res. 2016;2016:1632061
|
|
38.
|
Reactive sulfur species regulate tRNA methylthiolation and contribute to insulin secretion. Takahashi N, Wei FY, Watanabe S, Hirayama M, Ohuchi Y, Fujimura A, Kaitsuka T, Ishii I, Sawa T, Nakayama H, Akaike T, Tomizawa K.
|
|
39.
|
Effects of caloric restriction on O-GlcNAcylation, Ca(2+) signaling, and learning impairment in the hippocampus of ob/ob mice. Jeon BT, Heo RW, Jeong EA, Yi CO, Lee JY, Kim KE, Kim H, Roh GS. Neurobiol Aging. 2016 Aug;44:127-37.
|
|
40.
|
Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice. Jung MJ, Lee J, Shin NR, Kim MS, Hyun DW, Yun JH, Kim PS, Whon TW, Bae JW. Sci Rep. 2016 Jul 29;6:30887.
|
|
41.
|
Single ingestion of soy β-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects. Hashidume T, Kato A, Tanaka T, Miyoshi S, Itoh N, Nakata R, Inoue H, Oikawa A, Nakai Y, Shimizu M, Inoue J, Sato R. Sci Rep. 2016 Jun 17;6:28183.
|
|
42.
|
Paraventricular NUCB2/Nesfatin-1 Supports Oxytocin and Vasopressin Neurons to Control Feeding Behavior and Fluid Balance in Male Mice. Nakata M, Gantulga D, Santoso P, Zhang B, Masuda C, Mori M, Okada T, Yada T. Endocrinology. 2016 Jun;157(6):2322-32.
|
|
43.
|
Oxytocin Protects against Stress-Induced Cell Death in Murine Pancreatic β-Cells. Watanabe S, Wei FY, Matsunaga T, Matsunaga N, Kaitsuka T, Tomizawa K. Sci Rep. 2016 May 4;6:25185.
|
|
44.
|
Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Honma K, Hikosaka M, Mochizuki K, Goda T. Metabolism. 2016 Apr;65(4):482-91.
|
|
45.
|
Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Takemoto K, Doi W, Masuoka N. Biochim Biophys Acta. 2016 Apr;1862(4):647-50.
|
|
46.
|
Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Harada N, Hanaoka R, Horiuchi H, Kitakaze T, Mitani T, Inui H, Yamaji R. Sci Rep. 2016 Mar 10;6:23001.
|
|
47.
|
Pharmacological Inhibition of Monoacylglycerol O-Acyltransferase 2 Improves Hyperlipidemia, Obesity, and Diabetes by Change in Intestinal Fat Utilization. Take K, Mochida T, Maki T, Satomi Y, Hirayama M, Nakakariya M, Amano N, Adachi R, Sato K, Kitazaki T, Takekawa S. PLoS One. 2016 Mar 3;11(3):e0150976.
|
|
48.
|
GADD34-deficient mice develop obesity, nonalcoholic fatty liver disease, hepatic carcinoma and insulin resistance Naomi Nishio and Ken-ichi Isobe Sci Rep. 2015; 5: 13519.
|
|
49.
|
Patterns of Brain Activation and Meal Reduction Induced by Abdominal Surgery in Mice and Modulation by Rikkunshito Lixin Wang, Sachiko Mogami, Seiichi Yakabi, Hiroshi Karasawa, Chihiro Yamada, Koji Yakabi, Tomohisa Hattori, and Yvette Taché PLoS One. 2015; 10(9): e0139325.
|
|
50.
|
Hot water extracts of edible Chrysanthemum morifolium Ramat. exert antidiabetic effects in obese diabetic KK-Ay mice Yamamoto J, Tadaishi M, Yamane T, Oishi Y, Shimizu M, Kobayashi-Hattoria K. Bioscience, Biotechnology, and Biochemistry, Vol.79(7), 2015.
|
|
51.
|
Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARγ pathway. Horiba T, Katsukawa M, Mita M, Sato R. Biochem Biophys Res Commun. Vol.463(4), p846-52, Aug. 2015.
|
|
52.
|
Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and insulin resistance. Kim HY, Park SY, Lee MH, Rho JH, Oh YJ, Jung HU, Yoo SH, Jeong NY, Lee HJ, Suh S, Seo SY, Cheong J, Jeong JS, Yoo YH. J Hepatol. Vol.63(2), p477-85, Aug 2015.
|
|
53.
|
Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo. Kushibiki T, Okawa S, Hirasawa T, Ishihara M. Gene Ther. Vol.22(7), p553-9, Jul 2015.
|
|
54.
|
Preventive effects of astaxanthin on diethylnitrosamine-induced liver tumorigenesis in C57/BL/KsJ-db/db obese mice. Ohno T, Shimizu M, Shirakami Y, Miyazaki T, Ideta T, Kochi T, Kubota M, Sakai H, Tanaka T, Moriwaki H. Hepatol Res. Jul 2015
|
|
55.
|
Effects of liquid konjac on parameters related to obesity in diet-induced obese mice. Aoe S, Kudo H, Sakurai S. Biosci Biotechnol Biochem. Vol.79(7), p1141-6, Jul 2015.
|
|
56.
|
Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. FASEB J. Vol.29(6), p2397-411, Jun 2015.
|
|
57.
|
PRMT4 is involved in insulin secretion via the methylation of histone H3 in pancreatic β cells. Kim JK, Lim Y, Lee JO, Lee YS, Won NH, Kim H, Kim HS. J Mol Endocrinol.Vol.54(3), p315-24, Jun 2015.
|
|
58.
|
Hepatic NPC1L1 overexpression ameliorates glucose metabolism in diabetic mice via suppression of gluconeogenesis. Kurano M, Hara M, Satoh H, Tsukamoto K. Metabolism. Vol.64(5), p588-96, May 2015.
|
|
59.
|
Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. Kobori M, Takahashi Y, Akimoto Y, Sakurai M, Matsunaga I, Nishimuro H, Ippoushi K, Oike H, Ohnishi-Kameyama M. Journal of Functional Foods, Vol.15, p551–560, May 2015.
|
|
60.
|
Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice. Sun X, Yamasaki M, Katsube T, Shiwaku K. Nutr Res Pract. Vol.9(2), p137-43, Apr 2015.
|
|
61.
|
Insulin Release from the Beta Cells in Acatalasemic Mice Is Highly Susceptible to Alloxan-Induced Oxidative Stress. Kazunori Takemoto, Wakana Doi, Ken Kataoka, Kohji Ishihara, Da-Hong Wang., Hitoshi Sugiyama, Noriyoshi Masuoka. JDM, Vol.5 No.2, May 2015
|
|
62.
|
Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. Hu H, Guo Q, Wang C, Ma X, He H, Oh Y, Feng Y, Wu Q, Gu N. J Appl Toxicol. Mar 2015 .
|
|
63.
|
Compensatory hyperinsulinemia in high-fat diet-induced obese mice is associated with enhanced insulin translation in islets. Kanno A, Asahara S, Masuda K, Matsuda T, Kimura-Koyanagi M, Seino S, Ogawa W, Kido Y. Biochem Biophys Res Commun. Vol.13;458(3), p681-6. Mar 2015.
|
|
64.
|
Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo. Kushibiki T, Okawa S, Hirasawa T, Ishihara M. Gene Ther. Mar 2015.
|
|
65.
|
Compensatory hyperinsulinemia in high-fat diet-induced obese mice is associated with enhanced insulin translation in islets. Kanno A, Asahara S, Masuda K, Matsuda T, Kimura-Koyanagi M, Seino S, Ogawa W, Kido Y. Biochem Biophys Res Commun. Vol.458(3), p681-6, Mar 2015.
|
|
66.
|
Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. Quan X, Nguyen TT, Choi SK, Xu S, Das R, Cha SK, Kim N, Han J, Wiederkehr A, Wollheim CB, Park KS. J Biol Chem. Vol.290(7), p4086-96, Feb 2015.
|
|
67.
|
Endogenous Interleukin 18 Suppresses Hyperglycemia and Hyperinsulinemia during the Acute Phase of Endotoxemia in Mice. Yamashita H, Aoyama-Ishikawa M, Takahara M, Yamauchi C, Inoue T, Miyoshi M, Maeshige N, Usami M, Nakao A, Kotani J. Surg Infect (Larchmt). 2015 Feb;16(1):90-6.
|
|
68.
|
Hot water extracts of edible Chrysanthemum morifolium Ramat. exert antidiabetic effects in obese diabetic KK-Ay mice. Junpei Yamamoto, Miki Tadaishi, Takumi Yamane, Yuichi Oishi, Makoto Shimizu & Kazuo Kobayashi-Hattoria. Bioscience, Biotechnology, and Biochemistry, Published online: 10 Feb 2015
|
|
69.
|
Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. Hwang I, Park YJ, Kim YR1, Kim YN, Ka S, Lee HY, Seong JK, Seok YJ, Kim JB. FASEB J. Feb 2015.
|
|
70.
|
Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. Hu H, Guo Q, Wang C, Ma X, He H, Oh Y, Feng Y, Wu Q, Gu N. J Appl Toxicol. Mar 2015.
|
|
71.
|
Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Food Funct. Vol.6(1), p134-144, Jan 2015.
|
|
72.
|
Dietary nitrite supplementation improves insulin resistance in type 2 diabetic KKAy mice Ohtake K, Nakano G, Ehara N, Sonoda K, Ito J, Uchida H, Kobayashi J. Nitric Oxide, Vol.44, p31–38, Jan 2015.
|
|
73.
|
Salicornia herbacea prevents weight gain and hepatic lipid accumulation in obese ICR mice fed a high-fat diet. Pichiah PT, Cha YS. J Sci Food Agric. Dec 2014.
|
|
74.
|
Salacia reticulata has therapeutic effects on obesity. Shimada T, Nakayama Y, Harasawa Y, Matsui H, Kobayashi H, Sai Y, Miyamoto K, Tomatsu S, Aburada M. J Nat Med. Vol.68(4), p668-676, Oct 2014.Salicornia herbacea prevents weight gain and hepatic lipid accumulation in obese ICR mice fed a high-fat diet. Pichiah PT1, Cha YS. J Sci Food Agric. Dec 2014.
|
|
75.
|
Ghrelin administered spinally increases the blood glucose level in mice. Sim Y-B., Park S-H., Kim S-S., Kim C-H., Kim S-J., Lim S-M., Jung J-S., Suh H-W. Peptides, Vol.54, p162-165, Apr 2014.
|
|
76.
|
Chronic exposure to valproic acid promotes insulin release, reduces KATP channel current and does not affect Ca2+ signaling in mouse islets. Manaka K., Nakata M., Shimomura K., Rita RS., Maejima Y., Yoshida M., Dezaki K., Kakei M., Yada T. The Journal of Physiological Sciences, Vol.64(1), p77-83, Jan 2014.
|
|
77.
|
Impaired Lipid and Glucose Homeostasis in Hexabromocyclododecane-Exposed Mice Fed a High-Fat Diet. Yanagisawa R., Koike E., Win-Shwe TT., Yamamoto M. and Takano H. ENVIRONMENTAL HEALTH PERSPECTIVES, Jan 2014.
|
|
78.
|
Lipid-Lowering Effects of Pediococcus acidilactici M76 Isolated from Korean Traditional Makgeolli in High Fat Diet-Induced Obese Mice. Moon Y-J., Baik S-H. and Cha Y-S. Nutrients, Vol.6(3), p1016-1028, 2014.
|
|
79.
|
Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser1177/Thr497 of endothelial nitric oxide synthase in diabetic mice. Matsumoto S., Shimabukuro M., Fukuda D., Soeki T., Yamakawa K., Masuzaki H. and Sata M. Cardiovascular Diabetology, 13:30, 2014.
|
|
80.
|
Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. T.Tsuduki, I.Kikuchi, T.Kimura, K.Nakagawa, T.Miyazawa. Food Chemistry, Vol.139(1-4), p16-23, Aug 2013.
|
|
81.
|
Chronic treatment with novel GPR40 agonists improve whole-body glucose metabolism based on the glucose-dependent insulin secretion. H.Tanaka, S.Yoshida, H.Oshima, H.Minoura, K.Negoro, T.Yamazaki, S.Sakuda, F.Iwasaki, T.Matsui and M. Shibasaki. JPET, Jul 2013.
|
|
82.
|
Contribution of insulin signaling to the regulation of pancreatic beta-cell mass during the catch-up growth period in a low birth weight mouse model. Y.Yoshida, M.Fuchita, M.Kimura-Koyanagi, A.Kanno, T.Matsuda, S.Asahara, N.Hashimoto, T.Isagawa, W.Ogawa, H.Aburatani, T.Noda, S.Seino, M.Kasuga, Y.Kido. Diabetology International, Jul 2013.
|
|
83.
|
Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. T.Naito, A.Kuma and N.Mizushima. The Journal of Biological Chemistry, Jun 2013.
|
|
84.
|
Apelin Inhibits Diet-Induced Obesity by Enhancing Lymphatic and Blood Vessel Integrity. M.Sawane, K.Kajiya, H.Kidoya, M.Takagi, F.Muramatsu and N.Takakura. Diabetes, Vol.62(6), p1970-1980, Jun 2013.
|
|
85.
|
Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. S.Asahara, Y.Shibutani, K.Teruyama, H.Y.Inoue, Y.Kawada, H.Etoh, T.Matsuda, M.Kimura-Koyanagi, N.Hashimoto, M.Sakahara, W.Fujimoto, H.Takahashi, S.Ueda, T.Hosooka, T.Satoh, H.Inoue, M.Matsumoto, A.Aiba, M.Kasuga, Y.Kido. Diabetologia, Vol.56(5), p1088-1097, May 2013.
|
|
86.
|
Effects of hydrophilic statins on renal tubular lipid accumulation in diet-induced obese mice. K.Gotoh, T.Masaki, S.Chiba, H.Ando, K.Fujiwara, T.Shimasaki, Y.Tawara, I.Toyooka, K.Shiraishi, K.Mitsutomi, M.Anai, E.Itateyama, J.Hiraoka, K.Aoki, N.Fukunaga, T.Nawata, T.Kakuma. Obesity Research & Clinical Practice, May 2013.
|
|
87.
|
Amyloid-β Induces Hepatic Insulin Resistance In Vivo via JAK2. Y.Zhang, B.Zhou, B.Deng, F.Zhang, J.Wu, Y.Wang, Y.Le and Q.Zhai. Diabetes, Vol.62(4), p1159-1166, Apr 2013.
|
|
88.
|
Histidine augments the suppression of hepatic glucose production by central insulin action
Kimura K, Nakamura Y, Inaba Y, Matsumoto M, Kido Y, Asahara S, Matsuda T, Watanabe H, Maeda A, Inagaki F, Mukai C, Takeda K, Akira S, Ota T, Nakabayashi H, Kaneko S, Kasuga M and Inoue H.
Diabetes, Vol.62(4), p1003-1004, Apr 2013
|
|
89.
|
Improved transplantation outcome through delivery of DNA encoding secretion signal peptide-linked glucagon-like peptide-1 into mouse islets
Chae H Y, Lee M, Hwang H J, Kim H A, Kang J G, Kim C S, Lee S J, Ihm S-H.
Transplant International, Vol.26(4), p443-452, Apr 2013.
|
|
90.
|
Histidine augments the suppression of hepatic glucose production by central insulin action
K.Kimura, Y.Nakamura, Y.Inaba, M.Matsumoto, Y.Kido, S.Asahara, T.Matsuda, H.Watanabe, A.Maeda, F.Inagaki, C.Mukai, K.Takeda, S.Akira, T.Ota, H.Nakabayashi, S.Kaneko, M.Kasuga and H.Inoue. Diabetes, Mar 2013.
|
|
91.
|
Wogonin ameliorates hyperglycemia and dyslipidemia via PPARα activation in db/db mice without adverse side effects. Bak E-J, Kim J-H, Lee D-E, Choi Y-H, Kim J M, Woo G-H, Cha J-H, Yoo Y-J. Clinical Nutrition, Available online 26, Mar 2013.
|
|
92.
|
Extracellular Signal-Regulated Kinase in the Ventromedial Hypothalamus Mediates Leptin-Induced Glucose Uptake in Red-Type Skeletal Muscle. Toda C, Shiuchi T, Kageyama H, Okamoto S, Coutinho E A, Sato T, Okamatsu-Ogura Y, Yokota S, Takagi K, Tang L, Saito K, Shioda S and Minokoshi Y. Diabetes Mar 2013.
|
|
93.
|
Effect of vitamin E on alloxan-induced mouse diabetes. Kamimura W, Doi W, Takemoto K, Ishihara K, Wang D-H, Sugiyama H, Oda S, Masuoka N. Clinical Biochemistry, Mar 2013.
|
|
94.
|
Ablation of Rnf213 retards progression of diabetes in the Akita mouse. Kobayashi H, Yamazaki S, Takashima S, Liu W, Okuda H, Yan J, Fujii Y, Hitomi T, Harada K H, Habu T, Koizumi A. Biochemical and Biophysical Research Communications, Vol.432(3), p519-525, Mar 2013.
|
|
95.
|
Hypothalamic ATF3 is involved in regulating glucose and energy metabolism in mice. Lee Y-S, Sasaki T, Kobayashi M, Kikuchi O, Kim H-J, Yokota-Hashimoto H, Shimpuku M, Susanti V-Y, Ido-Kitamura Y, Kimura K, Inoue H, Tanaka-Okamoto M, Ishizaki H, Miyoshi J, Ohya S, Tanaka Y, Kitajima S, Kitamura T. Diabetologia, Mar 2013.
|
|
96.
|
Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. S. Asahara, Y. Shibutani, K. Teruyama, H. Y. Inoue, Y. Kawada, H. Etoh, T. Matsuda, M. Kimura-Koyanagi, N. Hashimoto, M. Sakahara, W. Fujimoto, H. Takahashi, S. Ueda, T. Hosooka, T. Satoh, H. Inoue, M. Matsumoto, A. Aiba, M. Kasuga, Y. Kido. Diabetologia, Feb 2013.
|
|
97.
|
Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Miura K, Yang L, Rooijen N, Brenner D A, Ohnishi H, Seki E. Hepatology, Vol.57(2), p577-589, Feb 2013.
|
|
98.
|
Transcriptional Regulatory Factor X6 (Rfx6) Increases Gastric Inhibitory Polypeptide (GIP) Expression in Enteroendocrine K-cells and Is Involved in GIP Hypersecretion in High Fat Diet-induced Obesity*. K.Suzuki, N.Harada, S.Yamane, Y.Nakamura, K.Sasaki, D.Nasteska, E.Joo, K.Shibue, T.Harada, A.Hamasaki, K.Toyoda, K.Nagashima and N.Inagaki. The Journal of Biological Chemistry, Vol.288, p1929-1938, Jan 2013.
|
|
99.
|
Improved hypothermic short-term storage of isolated mouse islets by adding serum to preservation solutions. Yasuko Kimura, Teru Okitsu, Liu Xibao, Hiroki Teramae, Atsuhito Okonogi, Kentaro Toyoda, Shinji Uemoto and Masanori Fukushima. Islets, Vol.5(1), Jan 2013.
|
|
100.
|
Anti-diabetic effect of amorphastilbol through PPARα/γ dual activation in db/db mice. Lee W, Ham J, Kwon H C, Kim Y K, Kim S-N. Biochemical and Biophysical Research Communications, Jan 2013.
|
![]()


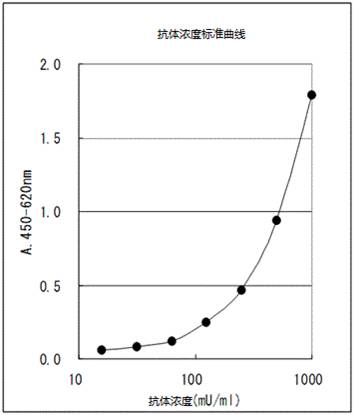


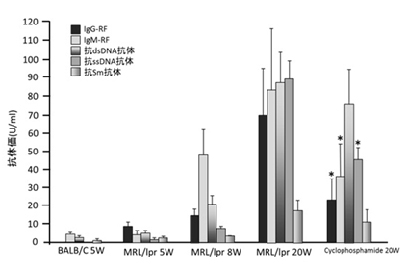


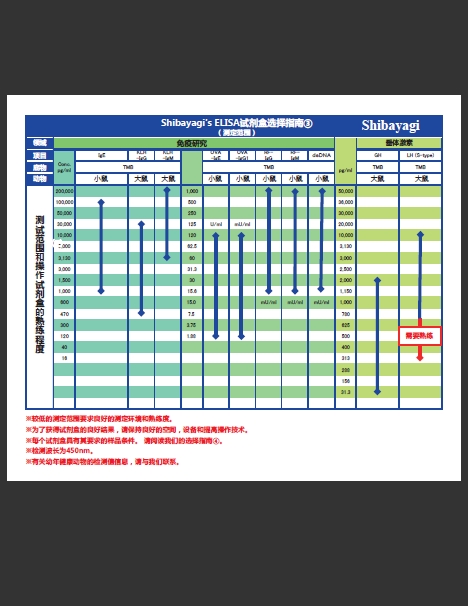













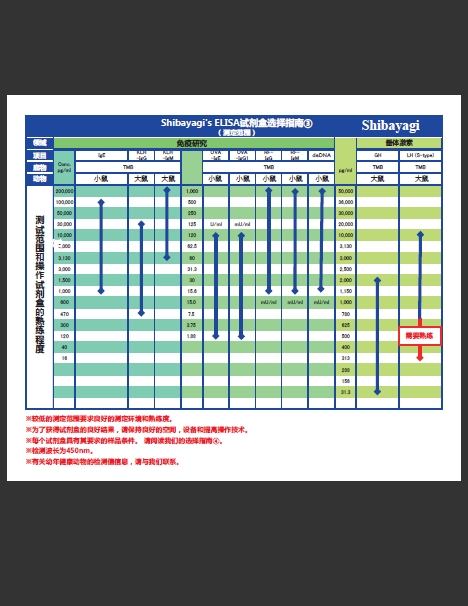


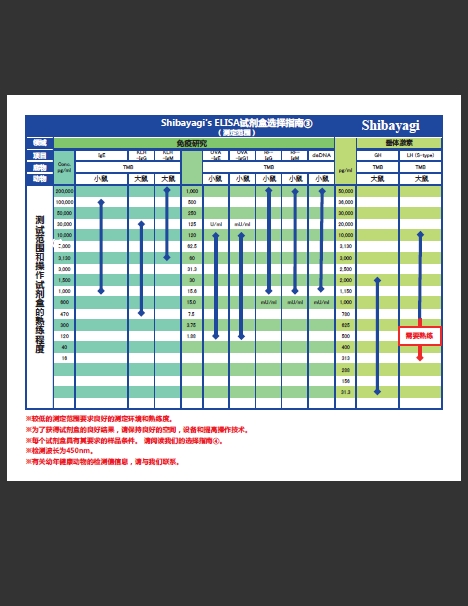


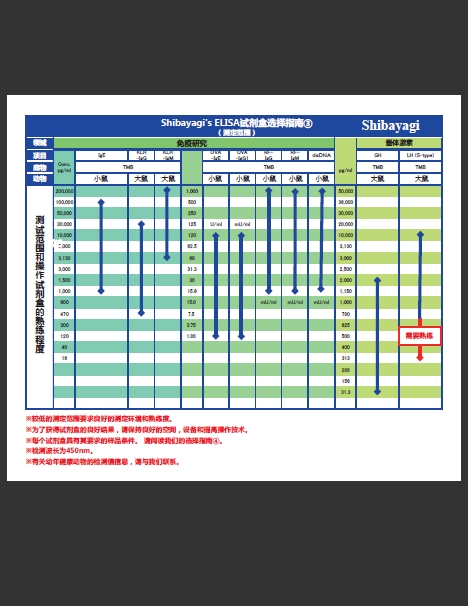


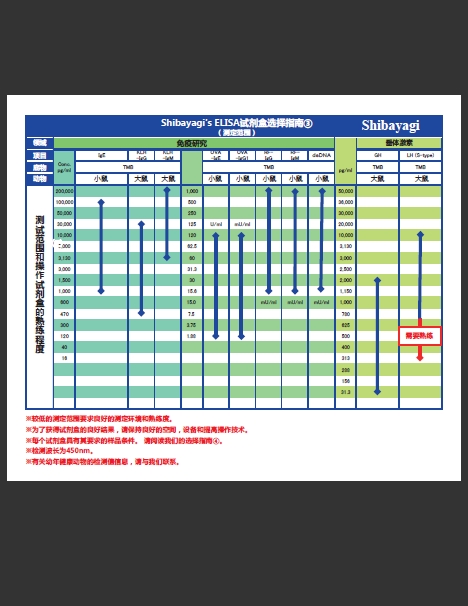
 增加3种产品!
增加3种产品!