Catalog ID
Offering the ability to scale-up to various automated bioreactors with increased speed for T cell culture, PRIME-XV T Cell CDM is fully optimized for immune therapy and research. Chemically defined and animal component-free, it delivers consistent growth while maintaining T cell functionality and potency in scalable therapy or research.
Details
PRIME-XV T Cell CDM is the first commercially available chemically defined, animal component-free medium for the expansion and cultivation of human T cells. The performance and functionality of PRIME-XV T Cell CDM continues to set the standard as the application process becomes more dynamic and closed.
Provides optimal performance:
- Supports vigorous T cell growth in static and dynamic automation systems while maintaining functionality
- Provides lot-to-lot consistency for reliable formulation composition
- Removes the effects of undefined components to minimize unwarranted T cell phenotypes
- Supports polarization to targeted T cell types such as Th1 and T regulatory cells to further possible therapy applications
Manufactured to facilitate transfer from research to clinic:
- Chemically defined, animal component-free formula minimizes risks from adventitious agents
- Manufactured in compliance with cGMP regulations
- Traceability documentation provided including Certificates of Analysis, Certificates of Origin, and a Drug Master File (DMF) filed with the US FDA
- Extensive QC testing including functionality, sterility, and endotoxin
- Mycoplasma USP <63> testing is standard on all lots
- Custom formulation, sizes and packaging available for all commercially available automation systems
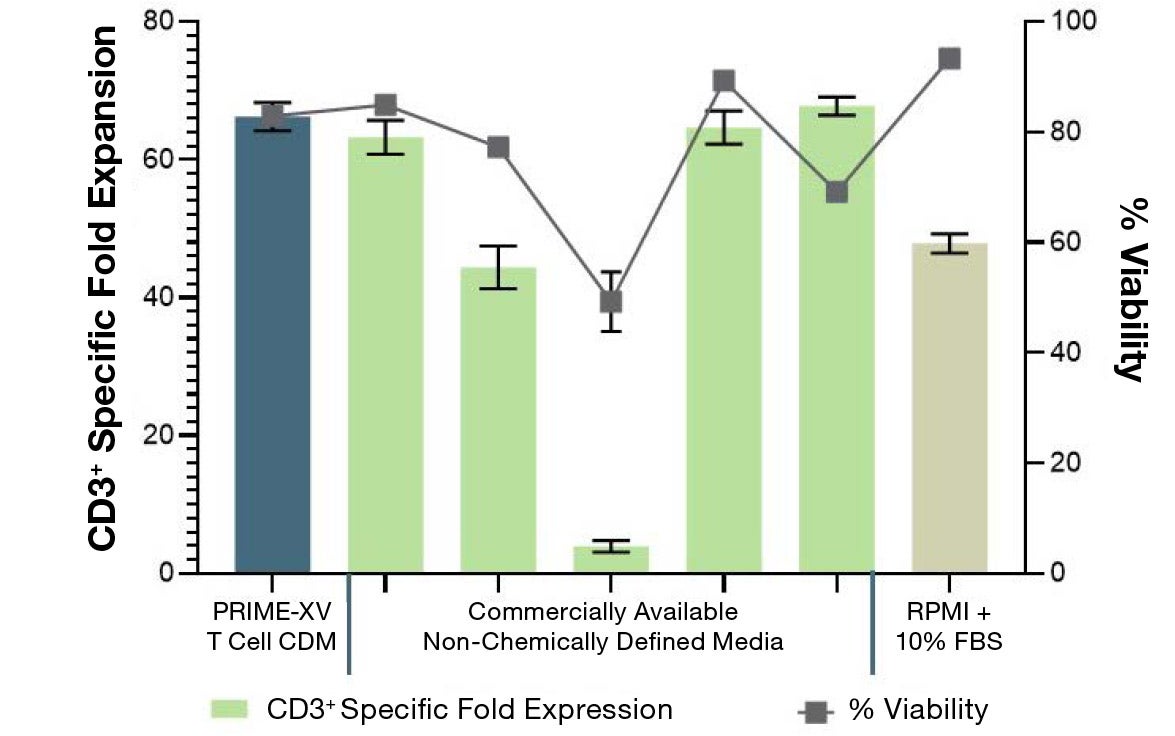
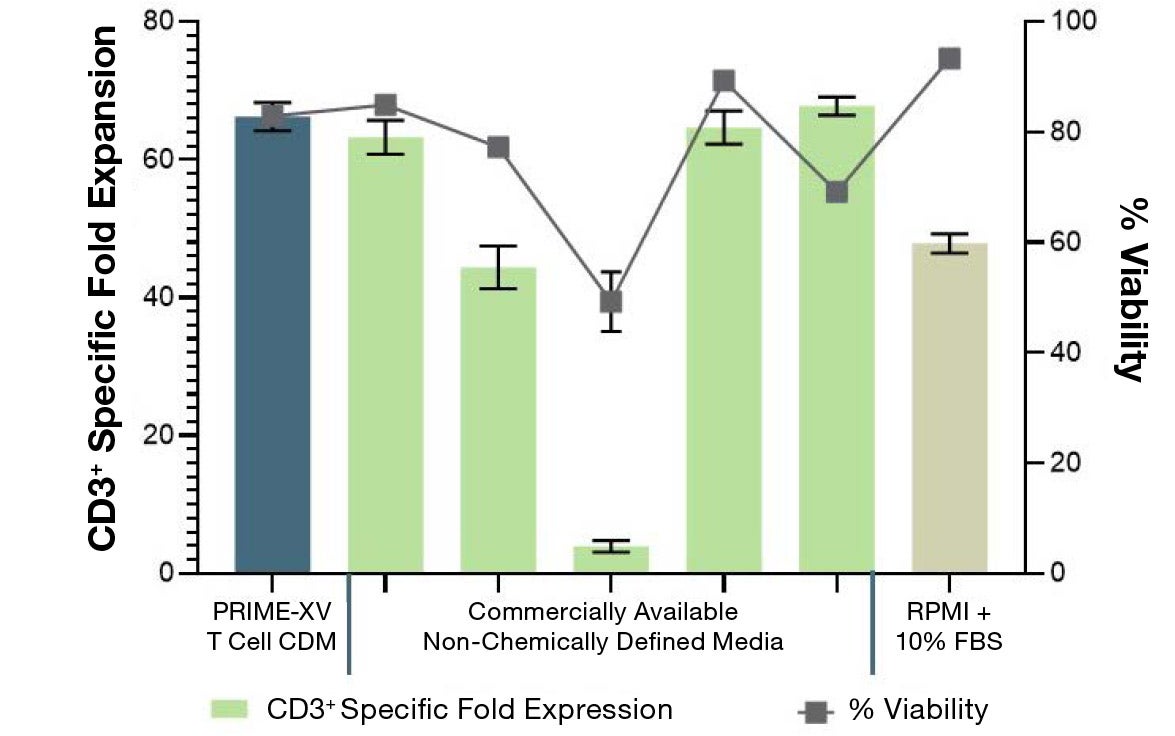
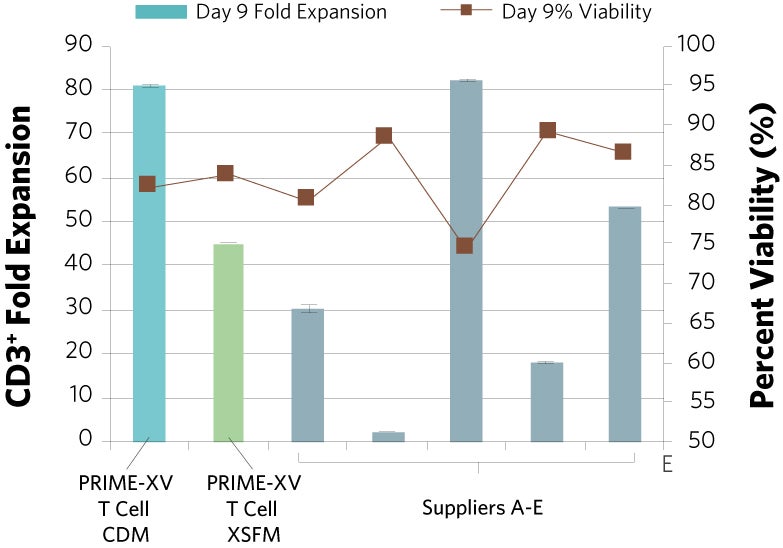
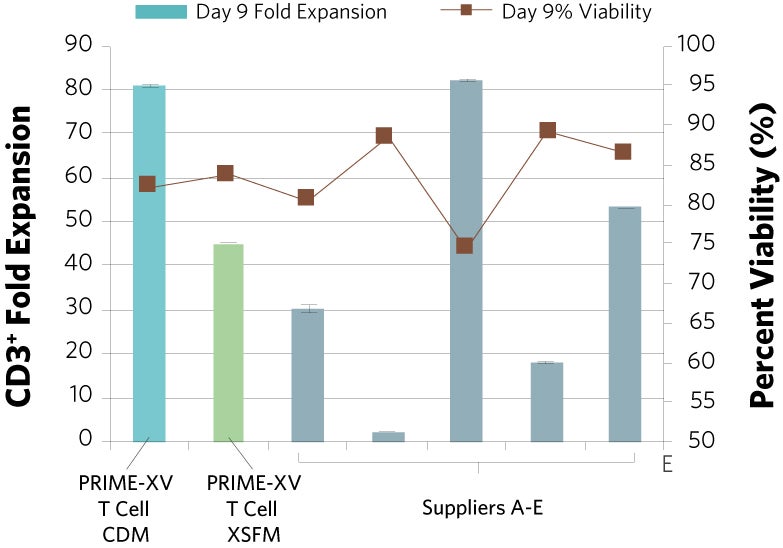
PRIME-XV T Cell CDM supports viable T cell expansion across different culture vessels
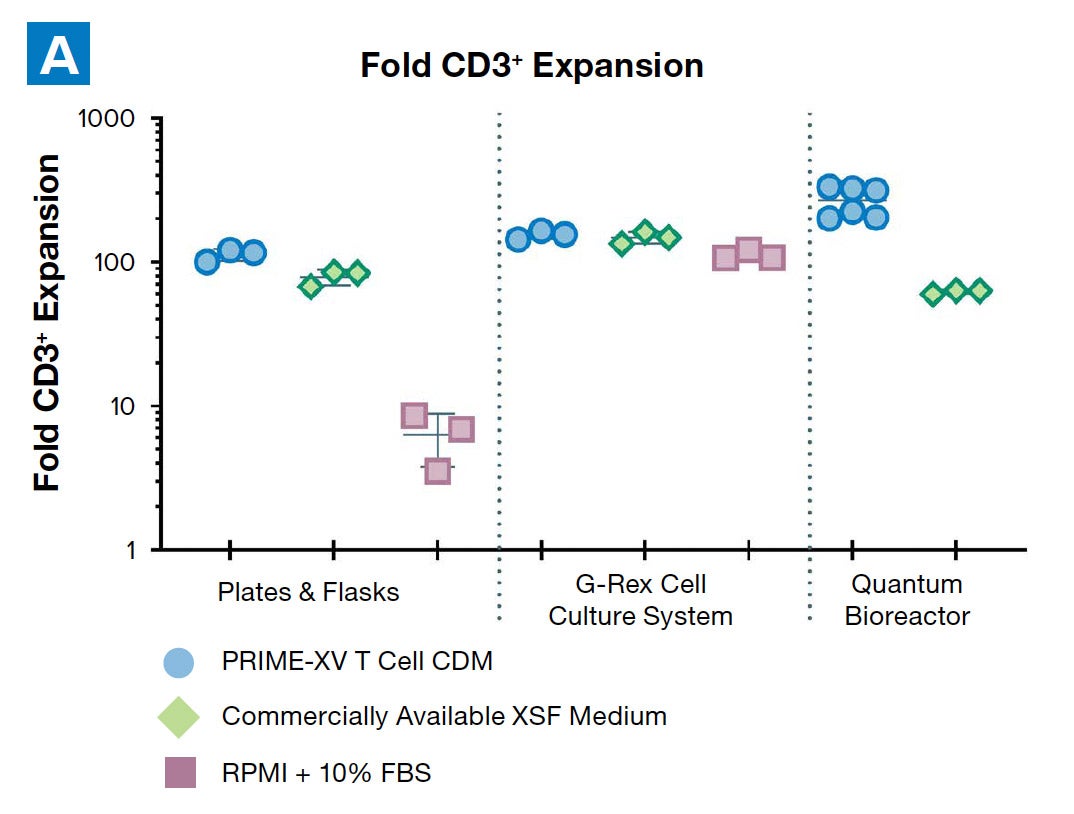
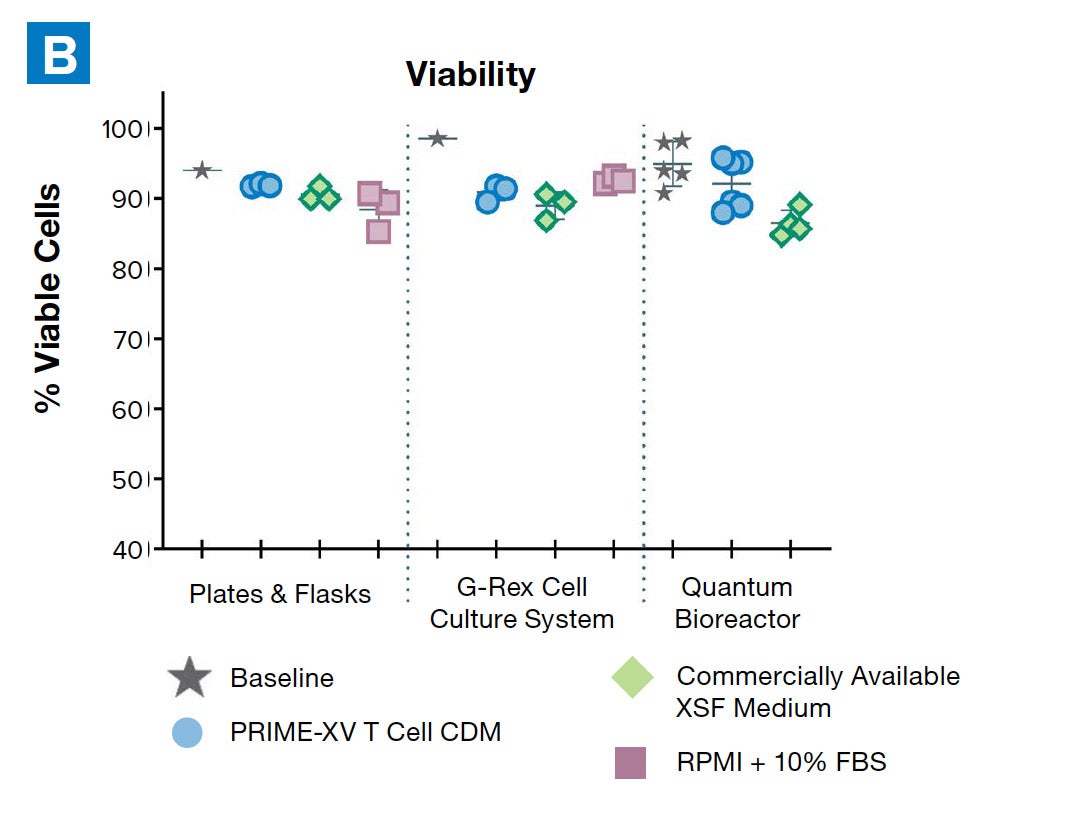
Figure 1: (A) Fold expansion of CD3+ cells in PRIME-XV T Cell CDM is robust across the three documented culture systems, performing as well as or better than FBS-supplemented RPMI and commercially available XSF media. (B) Viability is maintained at or above 85% by the end of the culture period in all three cell culture systems. Though significant inter-donor variability is observed, these data are representative of three donors, analyzed on day 14 (plates, flasks and G-Rex) or day 9 (Quantum bioreactors).
Publication, presentation, and protocol available for download
- Protocols support G-Rex and Quantum
- Protocols for plates and flasks can be provided

上海金畔生物代理FUJIFILM Irvine Scientific富士胶片欧文科技细胞培养基,部分现货,量多优惠,欢迎来电咨询18301939375!
FUJIFILM Irvine Scientific富士胶片欧文科技特色产品
BalanCD™ CHO Platform/培养基平台
BalanCD™ HEK293 培养基平台
PRIME-XV免疫细胞培养基
PRIME-XV干细胞培养基
冻存液和其它
免责声明
1. 本公司密切关注本网站发布的内容,但不保证发布内容的准确性、完整性、可靠性和最新性等。
2. 本公司不保证使用本网站期间不会出现故障或计算机病毒污染的风险。
3. 无论何种原因,使用本网站时给用户或第三方造成的任何不利或损害,本公司概不负责。此外,对于用户与其他用户或第三方之间因本网站发生的任何交易、通讯或纠纷,本公司概不负责。
4. 本网站可提供的所有产品和服务均不得用于人体或动物的临床诊断或治疗,仅可用于科研等非医疗目的。如任何用户将本网站提供的产品和服务用于临床诊断或治疗,以及其他特定的用途或行为,本公司概不保证其安全性和有效性,并且不负任何相关的法律责任。