Catalog ID 91154
Offering the ability to scale-up to various automated bioreactors with increased speed for T cell culture, PRIME-XV T Cell CDM is fully optimized for immune therapy and research. Chemically defined and animal component-free, it delivers consistent growth while maintaining T cell functionality and potency in scalable therapy or research.
PRIME-XV T细胞CDM提供了扩大到各种自动化生物反应器的能力,提高了T细胞培养的速度,完全优化了免疫治疗和研究。化学定义和不含动物成分,它提供一致的生长,同时保持T细胞的功能和可扩展治疗或研究的效力。
Details
PRIME-XV T Cell CDM is the first commercially available chemically defined, animal component-free medium for the expansion and cultivation of human T cells. The performance and functionality of PRIME-XV T Cell CDM continues to set the standard as the application process becomes more dynamic and closed.
PRIME-XV T细胞CDM是第一个商业上可用的化学定义的,无动物成分的培养基,用于扩增和培养人类T细胞。随着应用过程变得更加动态和封闭,PRIME-XV T Cell CDM的性能和功能继续设定标准。
Provides optimal performance:
- Supports vigorous T cell growth in static and dynamic automation systems while maintaining functionality
- Provides lot-to-lot consistency for reliable formulation composition
- Removes the effects of undefined components to minimize unwarranted T cell phenotypes
- Supports polarization to targeted T cell types such as Th1 and T regulatory cells to further possible therapy applications
Manufactured to facilitate transfer from research to clinic:
- Chemically defined, animal component-free formula minimizes risks from adventitious agents
- Manufactured in compliance with cGMP regulations
- Traceability documentation provided including Certificates of Analysis, Certificates of Origin, and a Drug Master File (DMF) filed with the US FDA
- Extensive QC testing including functionality, sterility, and endotoxin
- Mycoplasma USP <63> testing is standard on all lots
- Custom formulation, sizes and packaging available for all commercially available automation systems
PRIME-XV T Cell CDM supports viable T cell expansion across different culture vessels
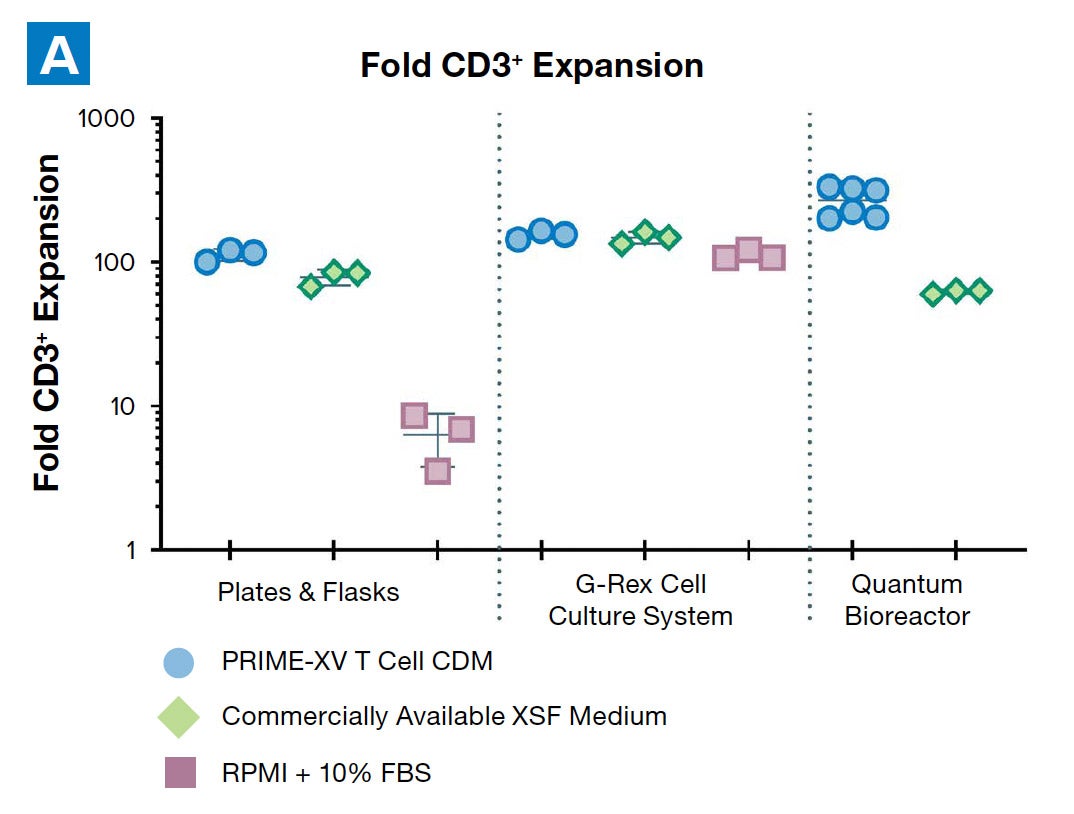
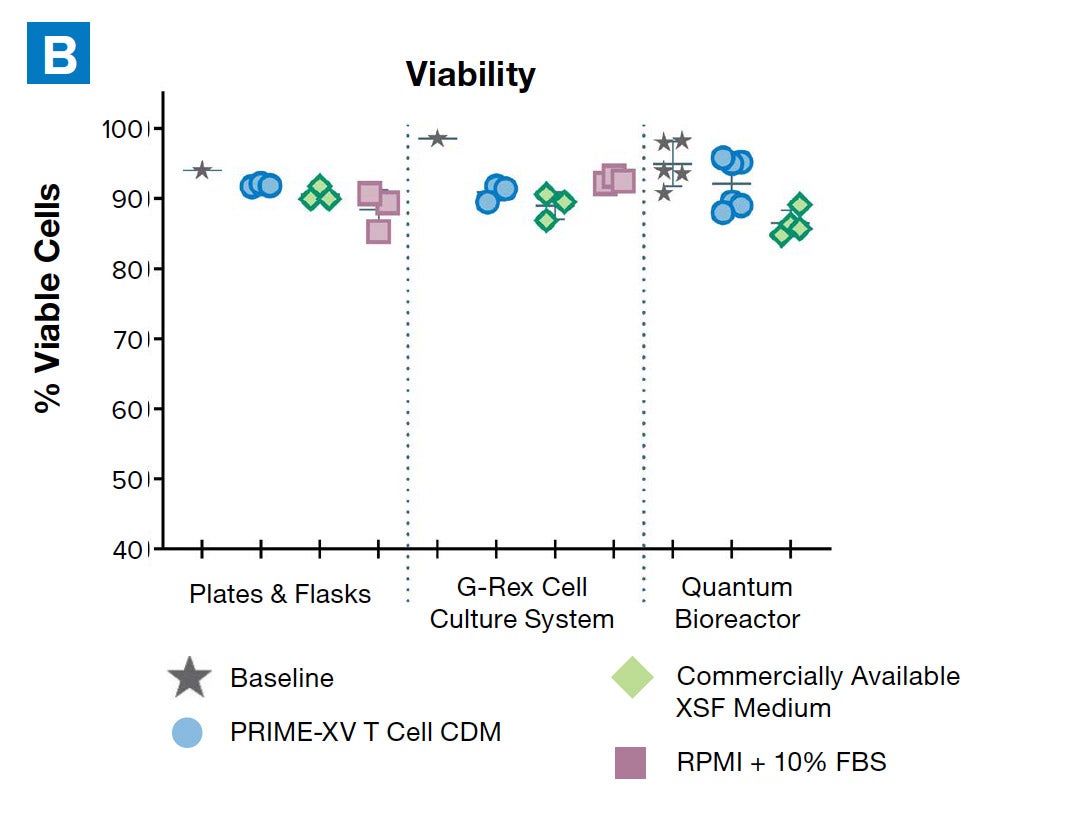
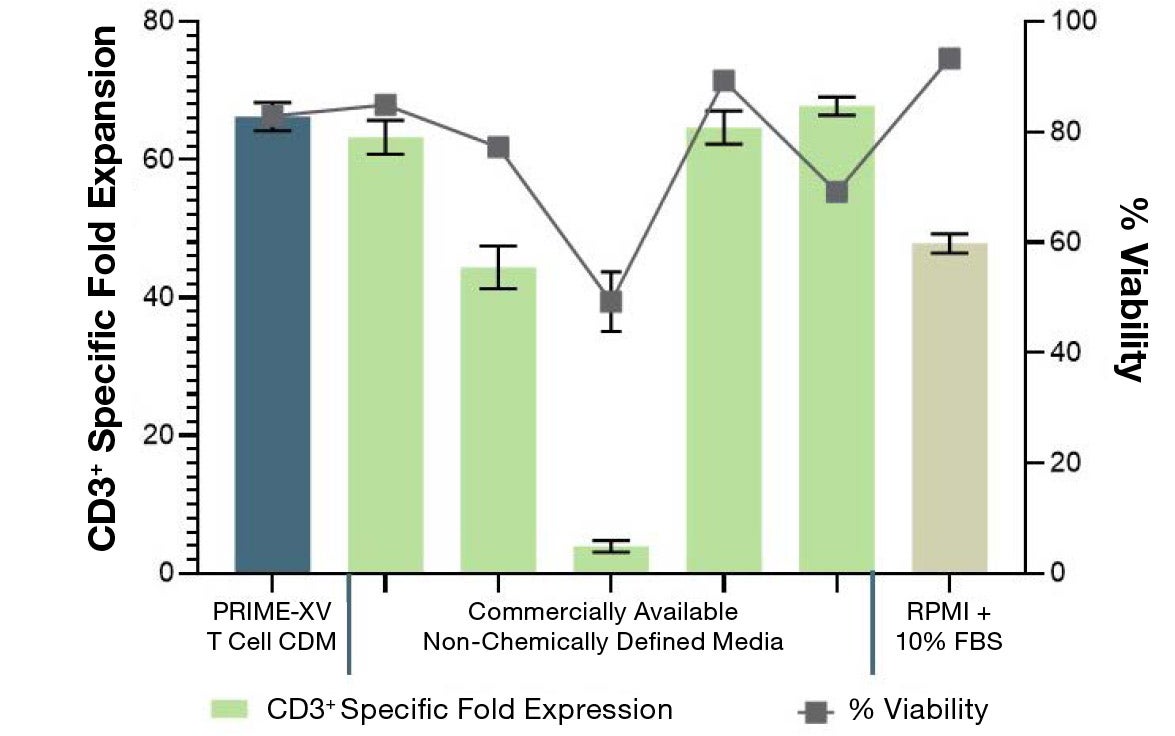
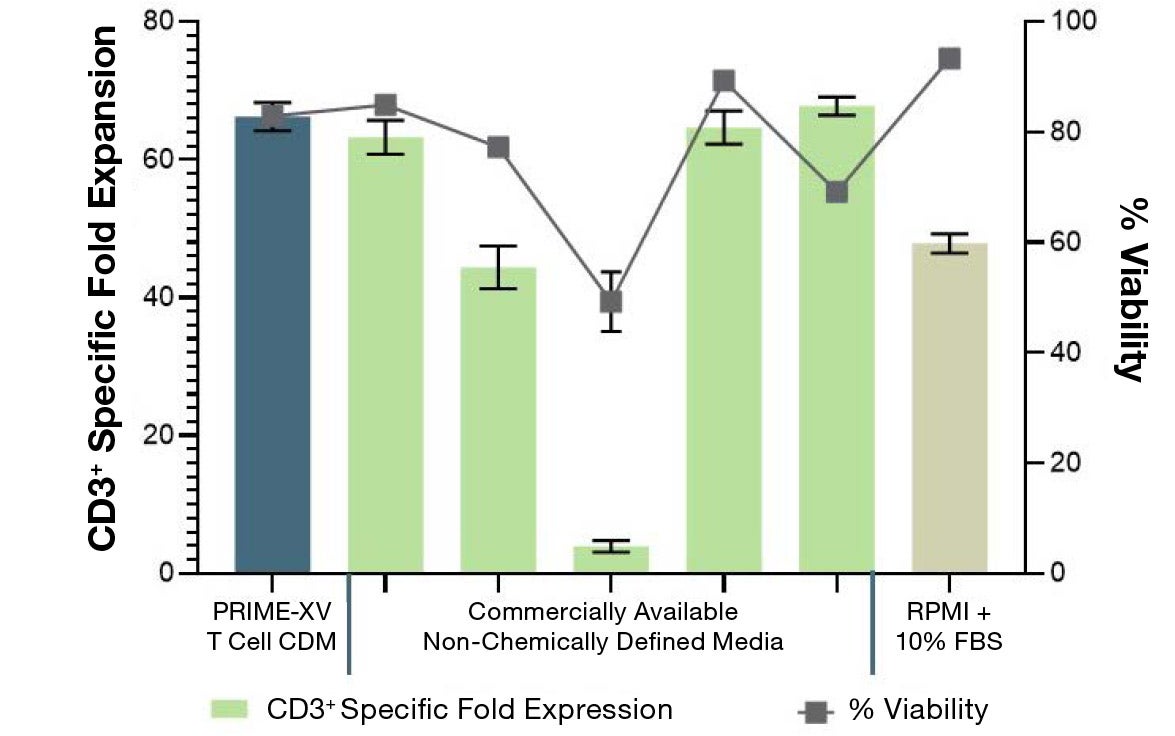
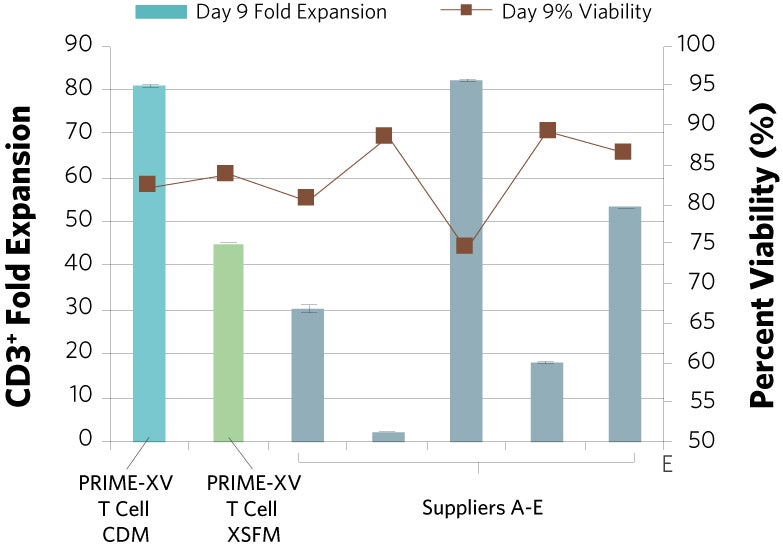
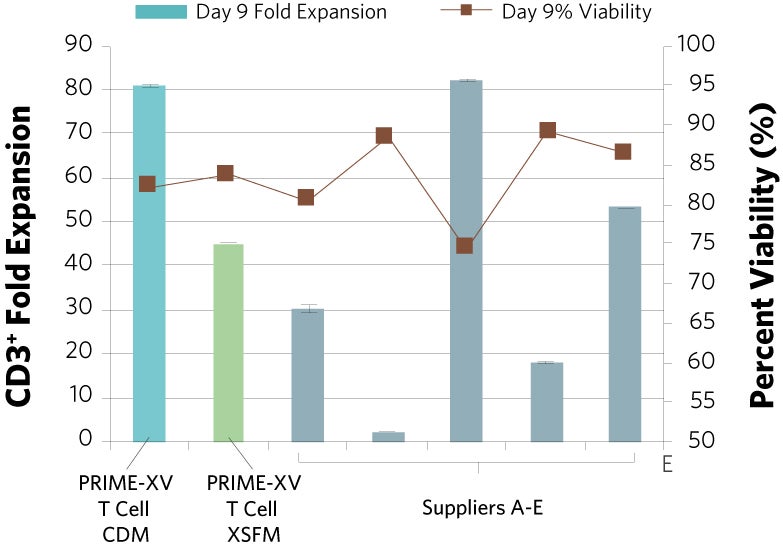
Figure 1: (A) Fold expansion of CD3+ cells in PRIME-XV T Cell CDM is robust across the three documented culture systems, performing as well as or better than FBS-supplemented RPMI and commercially available XSF media. (B) Viability is maintained at or above 85% by the end of the culture period in all three cell culture systems. Though significant inter-donor variability is observed, these data are representative of three donors, analyzed on day 14 (plates, flasks and G-Rex) or day 9 (Quantum bioreactors).
Publication, presentation, and protocol available for download
- Protocols support G-Rex and Quantum
- Protocols for plates and flasks can be provided